ins2outs –
integrated AI-powered Compliance Management System
Simplify compliance, amplify growth.
Why teams choose ins2outs?
From startups to enterprises, ins2outs makes compliance simple, scalable, and cost-effective — without the burden of manual processes.

Cut setup time by 75% with pre-built know-how sets.

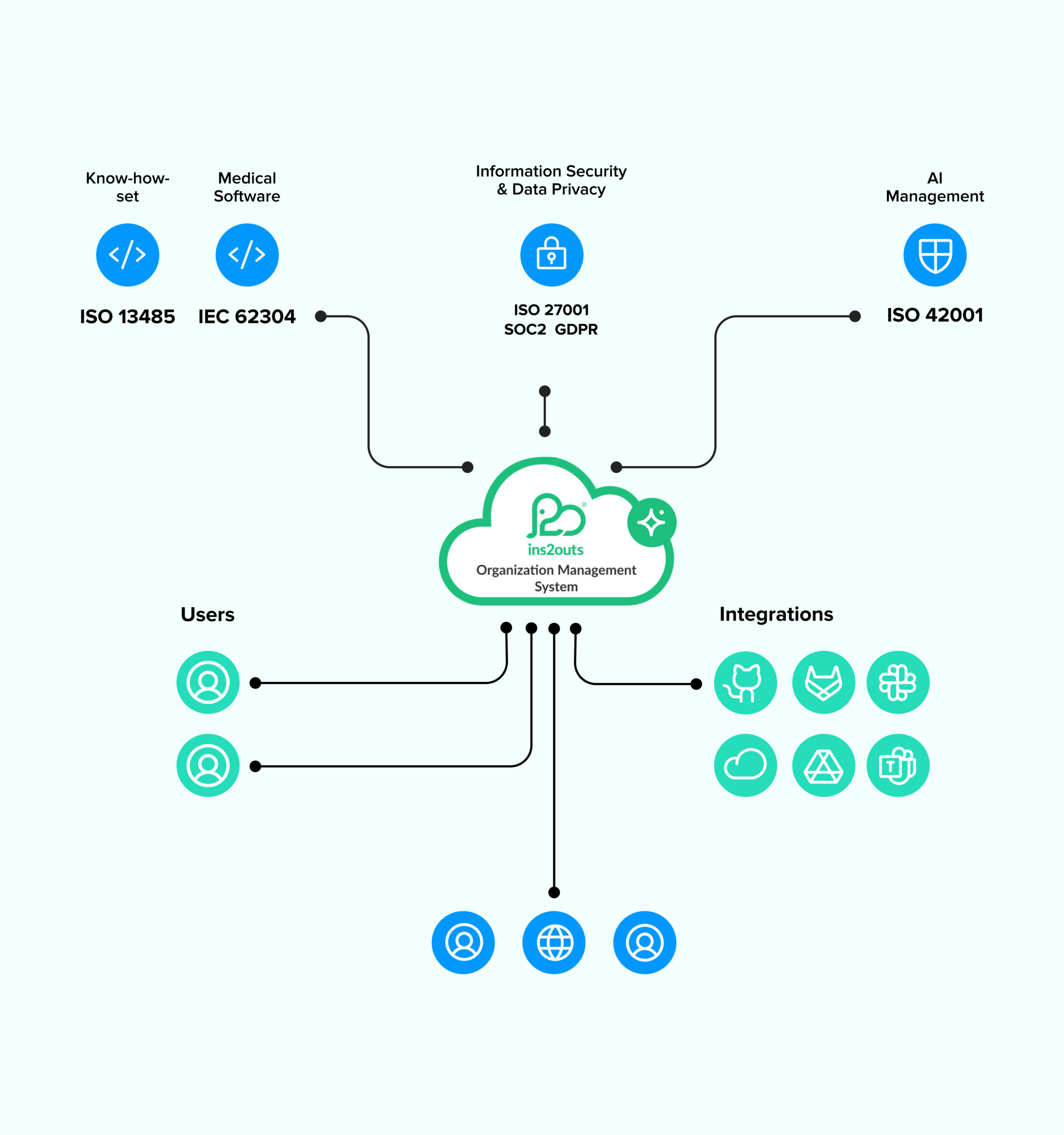
Audit-ready in 6 months for QMS, ISMS, AI governance, and more.

One platform, multiple standards & cross market requirements — ISO, FDA, MDR, IVDR.

Real-time compliance tracking with automated mapping.
Key Features – built for Compliance Leaders
All your compliance tasks, documents, and training — integrated and automated in one secure platform.
AI features roadmap
We’re progressively embedding AI across our compliance platform, starting with risk mapping and literature search today, followed by triage, template checks, and full validation.

AI Assistant
Create, summarize, review and identify gaps in your documents in seconds
Automate routine documentation with AI, focus on what is really important:
✓Create a document from scratch
✓ Summarize and polish
✓ Run gap assessment
Risk File Generation
AI maps risks based on device purpose and specs —
fast, structured, aligned with standardsPrecision risk calibration for confident compliance:
✓ Probability & Severity Scales
✓ Risk Acceptance Criteria
✓ Dynamic Risk Score Matrix
✓ Full Audit Traceability
AI-Driven Clinical Literature Rreview
Pulls relevant studies from PubMed, flags gaps, and surfaces insights to support CER
Automate and accelerate the process of gathering, analyzing, and justifying scientific literature to support your product development, regulatory submissions, and post-market surveillance.
✓ Describe your medical device
✓ Run AI literature search & analysis
✓ Get your report
Complaint Triage
Auto-categorizes complaints by urgency and context, recommends fixes using past data
Coming soon

CSOP Validation
Compares procedural steps to SOPs, flags deviations, and ensures hierarchy compliance
Coming soon

Template Checks
Verifies structure, catches missing or ambiguous content, and enforces guideline alignment
Coming soon
Pre-built know-how sets and SOPs
ins2outs provides ready-to-use frameworks that cut months off implementation time.
ISO 13485 + 21 CFR 820
Price: € 6,500 / set
Consulting services
Our experts act as an extension of your team — guiding you through every step of compliance and certification.
8 years of trusted Compliance Excellence
Compare & Win with ins2outs
Side-by-side, ins2outs delivers more speed, flexibility, and value than traditional compliance tools.

Pricing Made Simple
No surprises. Just clear, scalable plans that grow with your business.
30 days free trial included.










