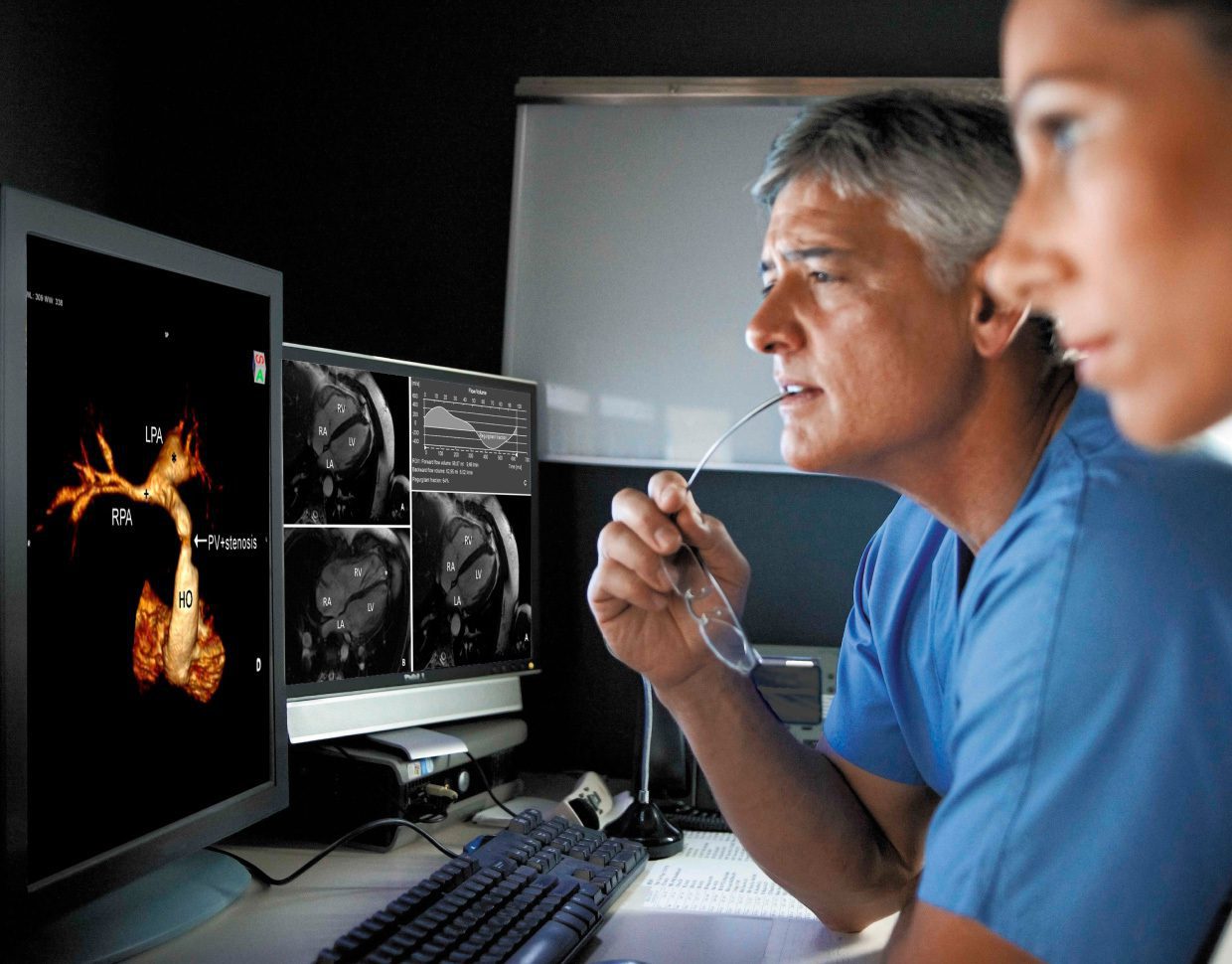
Medical devices, as highly regulated products, require all actors in the supply chain to adhere to specific regulatory conditions. The person responsible for regulatory compliance (PRRC) emerges as a key figure in ensuring these requirements are met, a topic that has seen extensive coverage on social media and medical device websites. A review of records in EUDAMED reveals a significant number of entities with a PRRC in place, highlighting the role's importance, yet also notes many that do not, indicating a gap in compliance.
As of December 2023, new MDCG documents have shed light on the PRRC's essential role, underlining the need for discussion. The mandate for a PRRC extends beyond EU manufacturers to include those from third countries. Products entering the EU from regions like the US, UK, Switzerland, or Asia must adhere to European standards, with an additional requirement for these entities to appoint an EU Representative (EU REP) who also has access to a PRRC.
The regulatory landscape for medical devices, including EUDAMED updates, potential amendments to the In Vitro Diagnostic Regulation (IVDR), Software as a Medical Device (SaMD), the AI Act, General Safety and Performance Requirements (GSPR), and more, is ever-evolving. This constant shift poses challenges for manufacturers in identifying applicable regulations and making timely, correct decisions to avoid non-certification, market withdrawal, or penalties.

Our expert regulatory team is dedicated to consulting and assisting clients with a variety of regulatory challenges. This includes defining or revising regulatory strategies, setting up quality management and information security systems, conducting internal audits, and providing training. We are committed to supporting your company in achieving its regulatory objectives. By acting as your PRRC, we can offer valuable contributions to your organization, facilitating quicker market access for your devices in a compliant manner.
Navigating the complex regulatory requirements of the medical device industry requires expert guidance. Our team stands ready to partner with you, ensuring your devices are not only compliant but also positioned for success in the global market. Contact us to learn more about how we can support your regulatory compliance efforts and accelerate your path to market.