At Star HealthTech, as with every other industry Star serves, we are passionate about improving people's lives with digital products that are a pleasure to use.
We're excited about what the future holds. Right now, we're working on projects that combine technologies like DTx, AI and telehealth with Design Thinking to help our clients ideate, co-create, launch, maintain and evolve breakthrough solutions that will make healthcare more accessible, affordable and human-centered.
This has been a strong year of growth. While expanding our client base and deepening our ongoing partnerships, we acquired a MedTech company in Europe that's helped bolster our SaMD (software as a medical device), MedTech, medical device and digital healthcare capabilities.
Our HealthTech Practice has also seen the birth of two new industry sub-categories: regulatory consulting and interoperability. As an end-to-end innovation consultancy, this offering enables us to serve our clients further upstream and in new ways while furthering our expertise.
In short, it's been a bold, busy year. And next year, we're even more ambitious.
HealthTech trends
The medical device industry continues to boom globally. It is projected to grow at 5.6% CAGR from $605B in 2020 to $796.5B in 2021. Worldwide, the promise of technology to transform all aspects of care delivery, including non-clinical applications at the digital front door, have helped increase accessibility and engagement.
We forecast the acceleration of digital implementation in the marketplace to pick up speed as more companies invest in their tech footprint. This will bear results both in the development of new products and the ongoing modernization of existing tools.
What companies are learning is that it’s never too late to get started or go further on their digital journey. That’s why we are particularly focused on SaMD, interoperability and regulatory consulting as three closely interconnected pieces of the MedTech and digital healthcare puzzle that businesses can leverage to increase revenue opportunities while improving user experience and engagement.
Learn key insights needed to build a better MedTech products and SaMD solutions.
Software as a medical device
Between 2019 and 2026, SaMD is projected to expand at a 69.3% CAGR. Outside of pharma and drug development, it is among the fastest-growing segments of the healthcare industry.
SaMD comprises technology intended to diagnose, mitigate, treat, cure or prevent diseases and directly benefits patients, providers, healthcare companies and their investors. At its core, we see rapid data collection as one of its greatest assets. For example, monitoring changes in medical conditions can be quickly assessed to promote better outcomes. This same data can encourage a patient to seek treatment earlier, putting less burden on the healthcare system. Concurrently, businesses can use data to refine their products, develop new ones or find other forms of monetization (with patient privacy and consent, of course).
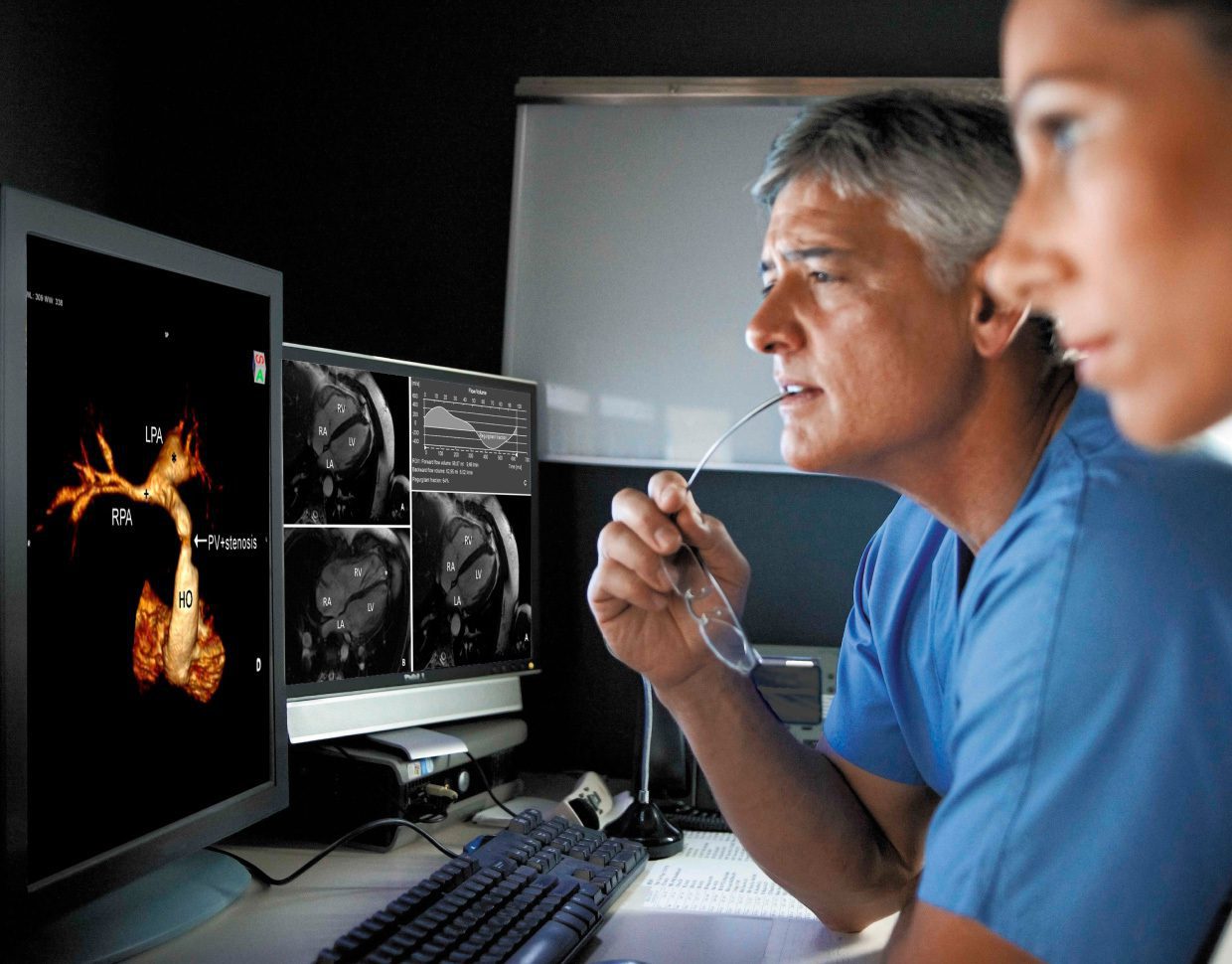
SaMD is playing many roles in healthcare. There is massive potential in SaMD’s ability to facilitate Clinical Decision Support or go even further in directly making diagnoses. The technology is already there in some cases. We have an ongoing partnership with a European teledermatology company that can assess skin lesions as benign, premalignant and malignant along with melanoma. A process that used to take weeks in getting a diagnosis can now be done almost instantaneously.
There are opportunities like this to make an impact throughout the healthcare landscape. The question we help our clients answer is whether they want to create a Clinical Decision Support tool or a SaMD that directly makes the diagnosis which will be classed higher. Then we help them design and build it.

Interoperability
In the world of connected healthcare, it’s not enough to create an excellent solution. Your device must also communicate, exchange data and be a part of the greater ecosystem. There are abundant opportunity spaces around interoperability. It can be approached from various angles, including new product development, modernizing legacy devices and the creation and implementation of interoperability strategies to help companies connect their software to other systems and offer seamless interphases.
With the passage and enactment of the 21st Century Cures Act, interoperability has shifted from a nice to have to a must through industry standardization via FHIR (Fast Healthcare Interoperability Resources) and HL7. Naturally, this hugely impacts new product development. At Star, we’re helping companies go beyond meeting regulatory requirements to truly unlock the value of interoperability. The advantages include better protection of patient health and other sensitive data, improved user experience, increased efficiency and mitigated provider burnout.
The advantage of FHIR is its ability to work across legacy systems. But this is also a moment for legacy healthcare businesses to modernize and leverage their scale in new ways. While not exactly a case study for interoperability, we like to draw comparisons to our work with ZEISS. It is a 175-year-old company that has long been a leader in the field of optics. We co-created with them the award-winning VISU360 telehealth solution. The result has been increased revenue, decreased overhead and greater patient accessibility. Helping legacy healthcare businesses embrace new technologies and modernize especially through the lens of interoperability provides the same advantages.
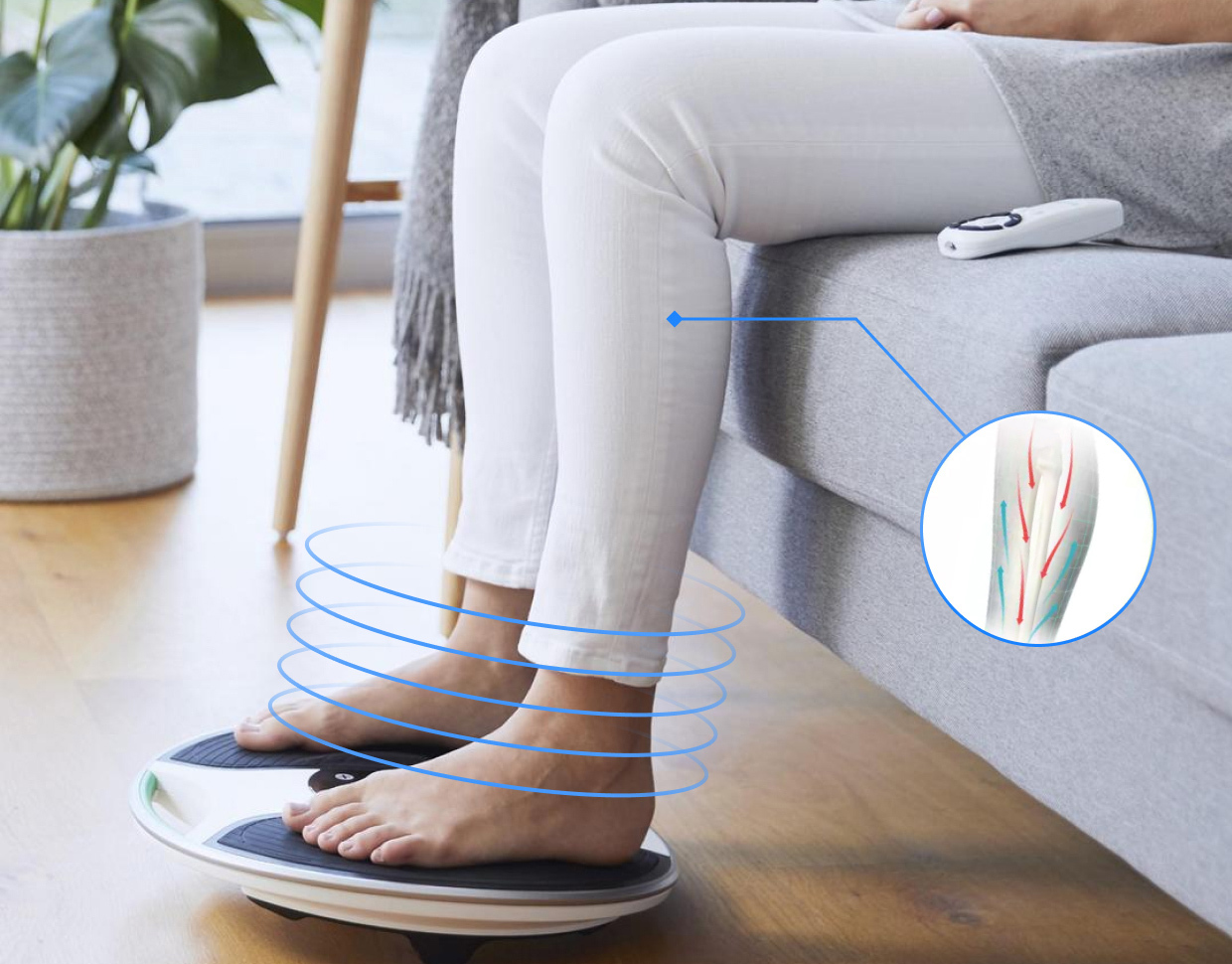
Likewise, we see potential in the creation of interoperable platforms that connect the varied IoMT devices in the market and synthesize all this data into something understandable and actionable for caregivers. For example, we are currently working with a European MedTech company that’s helping to enable aging in place. With their platform, caregivers can track the health status of patients through in-home sensors, wearables and other remote monitoring devices.
The Class III medical device also uses AI to synthesize millions of data points into concise updates and alerts, empowering caregivers with the right information at the right time. We built the architecture to be infinitely scalable so that it will adapt even as new technologies emerge. Platforms like these will be essential across every demographic and region as more SaMD and digital healthcare devices come into the market.

Regulatory consulting
Regulatory consulting ties together everything our HealthTech Practice works on. Even if a company isn’t ready to start their regulatory journey, this might be an objective down the road. What we help our clients understand is which approaches are best for their current and future needs, and how they can prepare accordingly.
Anybody interested in SaMD development, however, will need to understand the approval process from day one. Despite its challenges, we see the stricter regulatory burden on SaMD as an opportunity. First and foremost, it exists to ensure medical devices are safe and effective, which is how you gain payer support. Likewise, since not everyone is ready to invest time and resources into the regulatory process, this means you can limit your competition, especially if they can’t use your product as a predicate device under FDA 510(k).
With our consulting, know-how sets and deep expertise in medical device development, we can help our clients move seamlessly through the regulatory journey. As more companies rush to get into this space to differentiate themselves from consumer-grade healthcare technology solutions, they’ll benefit from our extensive experience bringing numerous medical devices from ideation to market.
Looking ahead: 2022 and beyond
The outlook for the HealthTech industry is incredibly promising over the next ten years. In 2022, we will continue to grow our HealthTech Practice and enhance our portfolio of SaMD, medical device and digital healthcare projects. We’re similarly looking forward to further increasing our regulatory consulting and interoperability business.
While every year is a building year at Star, we’re now in an even stronger position to make bolder moves. Above all, we’re excited about helping our clients accelerate their digital journey to develop even more impactful products that improve outcomes throughout healthcare.
Be inspired. Be endgame-driven. Shine with Star.